the results at the end of a test or trial|What happens at the end of a trial? : Tuguegarao Reporting and using trial results. Go to: 1. Planning communications. It is important to communicate the progress of a trial, . In this games collection, you'll find a variety of fun chess games to play. If you're looking to play against other chess players online, the most popular online chess game is Master Chess. Another popular chess game with a multiplayer game mode is Spark Chess. Practice Chess. Chess is a skill game where practice
the results at the end of a test or trial,6 years ago by Clara Team • 5 min read. After a clinical trial is completed, the research team carefully analyzes information collected during the study to make decisions about the findings and any need for further testing, but the next steps can vary based .After a clinical trial ends, the results are often peer reviewed before being .What happens at the end of a trial? Bias in clinical trials may also affect the results-based merit of a trial. Bias may threaten the internal validity of a trial and may generate erroneous conclusions. Potential sources .
During the “End of Study” stage, is when research participants want to learn more about what the study found and how they contributed to the findings. Find out what really matters to the study population through regular . Reporting and using trial results. Go to: 1. Planning communications. It is important to communicate the progress of a trial, .

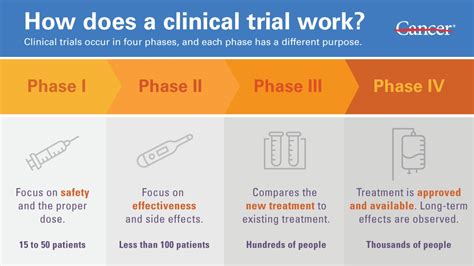
There are three main phases in a clinical trial — and sometimes there is a fourth, or post-market, phase that studies and assesses the long-term safety and effectiveness of the drug. This phase .how safe it is. whether it has any side effects. whether it is better than the current treatment (or placebo) how much, or how often a treatment should be used. Researchers have a . Most trials do not release interim summaries on efficacy and toxicity of the experimental treatments being tested, with this information only released to the public .the results at the end of a test or trial Post-trial care should be offered to varying degrees at the end of every trial, consistent with the principles of ethical research. Distinguishing these concepts prevents . If the results are published, the two main ways to access the results of a clinical trial are to: Find them online from publicly available databases. Contact the company that sponsored the clinical trial. Regardless of what the results are, the outcome of a clinical trial should be published so that others can use the information to help them . We report the primary analysis results of this ongoing pivotal phase 3 trial. . For analysis of the primary end point, the trial was designed for the null hypothesis that the efficacy of the . Primary efficacy analysis demonstrates BNT162b2 to be 95% effective against COVID-19 beginning 28 days after the first dose; 170 confirmed cases of COVID-19 were evaluated, with 162 observed in the placebo group versus 8 in the vaccine group Efficacy was consistent across age, gender, race and ethnicity demographics; observed . There are three main phases in a clinical trial — and sometimes there is a fourth, or post-market, phase that studies and assesses the long-term safety and effectiveness of the drug. This phase only occurs if the drug is approved by FDA. And sometimes there are in-between phases, for example, Phase 1a or a Phase 2b. 1. Trial design and registration: Clinical trials are carefully designed; the protocol 1 for conducting the trial and the statistical analysis plan (SAP) detailing the planned data analyses are developed well before the first participant is enrolled. The protocol and the SAP constitute some of the most important metadata of the trial. During .What happens to a treatment after the trial results are analysed depends on the phase of the trial. After a Phase 1 or 2 trial the results will tell the researchers whether to move on to the next phase or to stop testing because the drug was unsafe or ineffective. When a Phase 3 trial is complete, the researchers examine the data and decide .
Crossword Answers: A test or trial (10) RANK. ANSWER. CLUE. EXPERIMENT. A test or trial (10) PROOF. A test or trial; a standard strength of alcoholic spirits; factual evidence; or, a preliminary impression of a page for review by a copy-editor (5) Advertisement.The termination or suspension may apply to all clinical trials at a CRS or those specific to a PI/IoR. If an RE/RA terminates or entirely suspends a clinical trial, DAIDS will communicate this to all participating CRSs. If the clinical trial is terminated or suspended at a particular CRS, then the RE/RA may contact the PI/IoR/CRS directly.
B.1 Introduction. According to Meinert (1986) any investigator who undertakes a trial has a responsibility to make the results obtained from it available for public scrutiny via a published manuscript as soon after the results have been obtained as possible. The goal in the publication should be to provide a clear, concise and accurate descrip . What happens at the end of a trial when a patient responds to an investigational medication and benefits considerably? Many people believe that this patient should continue to receive the beneficial drug. This belief underlies the idea of post-trial access—providing investigational interventions post-trial to participants who benefited . The Oxford/AstraZeneca vaccine is nearing the end of phase 3, and the research team has released interim figures from the trial. These are basically a sneak peek at how the testing is going, to . An Overview of Phase II Clinical Trial Designs. Clinical trials are studies to test new treatments in humans. Typically, these treatments are evaluated over several phases to assess their safety and efficacy. Phase I trials are designed to evaluate the safety and tolerability of a new treatment, typically with a small number of patients (e.g .A necessary companion to well-designed clinical trial is its appropriate statistical analysis. Assuming that a clinical trial will produce data that could reveal differences in effects between two or more interventions, statistical analyses are used to determine whether such differences are real or are due to chance. Data analysis for small clinical trials in .

Any particular performance of a random experiment is called a trial. By Experiment or Trial in the subject of probability, we mean a Random experiment unless otherwise specified. Each trial results in one or more outcomes . Examples . Tossing 4 coins ; Picking 3 balls from a bag containing 10 balls 4 of which are red and 6 blue ; Rolling a die
Diagnostic tests (such as genetic or imaging tests) are health interventions used to determine the existence or severity of a disease (Sun et al. 2013; Huang et al. 2017).The development and introduction process of diagnostic tests is equivalent to the development of other health technologies such as therapeutic drugs, and similarly the . Several factors can influence the decision to terminate an ongoing clinical trial including ethical concerns, alterations in accepted clinical practice that make the continuation of a clinical trial unwise, and/or reaching a positive or negative statistical end point earlier than anticipated (Table).The discontinuation of a clinical trial can be .
the results at the end of a test or trial What happens at the end of a trial? Most trials do not release interim summaries on efficacy and toxicity of the experimental treatments being tested, with this information only released to the public after the trial has ended.
Phase 1 trials try to determine dosing, document how a drug is metabolized and excreted, and identify acute side effects. Usually, a small number of healthy volunteers (between 20 and 80) are used .
the results at the end of a test or trial|What happens at the end of a trial?
PH0 · What happens when a clinical trial is completed
PH1 · What happens at the end of a trial?
PH2 · The effects of releasing early results from ongoing clinical trials
PH3 · Reporting and using trial results
PH4 · Post
PH5 · Interpreting Results of Clinical Trials: A Conceptual Framework
PH6 · How to end a clinical trial
PH7 · How To Find the Results of a Clinical Trial or Study
PH8 · How To Find the Results of a Clinical Trial or Study
PH9 · How Clinical Trials Work: From Start to Finish
PH10 · End of Study